Products
Product pages available in
EN - DE - FR - ES
Sustainability
News, events and stories
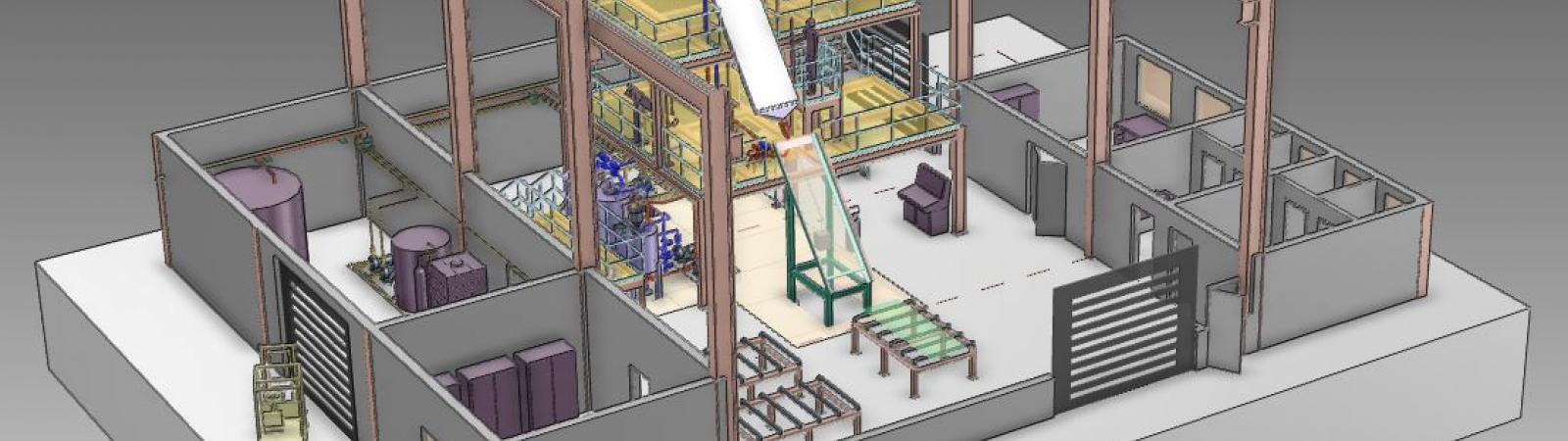
One of the steelmaking paths ArcelorMittal is exploring involves using ‘clean’ or non-carbon sources of energy. For example, hydrogen or electrolysis can be used for the direct reduction of iron ore. By avoiding the need for carbon in the steelmaking process, CO2 emissions can be reduced permanently.
H2 Hamburg: producing steel with hydrogen
In 2019, ArcelorMittal Hamburg launched a new project to explore the use of hydrogen for the direct reduction of iron ore in the steel production process. The Hamburg plant is already one of the most efficient in the ArcelorMittal group as it uses natural gas in the direct reduction of iron ore.
The new hydrogen-based process can produce steel with lower CO2 emissions than any existing production process. The process separates very pure hydrogen (+95%) from the top gas of the existing plant. The gas is then used in the direct reduction of iron.
A cooperation agreement has been signed with the University of Freiberg (Germany) which will see researchers test the new procedure at the Hamburg plant. Initially grey hydrogen (less than 95% purity) will be used to assess the process and produce around 100,000 tons of direct reduced iron annually. ArcelorMittal has already allocated €65 million for the project.
The plant should also be able to run on green hydrogen (generated from renewable sources) when it becomes available in sufficient quantities. In the future, a pilot plant may be built to prove that the technology is feasible at an industrial scale.
Siderwin: Replacing carbon with electricity
Launched in October 2017, Siderwin is a radically new iron production process which uses electrolysis technology to produce steel without CO2 emissions. The Siderwin project brings together 12 European partners and follows on from an initial project launched in the early 2000s which explored the use of electrolysis to produce steel at the laboratory level.
The initial project proved that electrolysis can be used to produce a few kilos of iron. Siderwin changes the scale. A large pilot unit will be installed at ArcelorMittal’s Maizières (France) research campus and should be able to produce up to a hundred kilograms of iron metal.
Electrolysis technology has the advantage of flexibility. That means the iron metal production process can be interrupted, a key benefit in facilitating the incorporation of intermittent renewable energy into the electricity grid. Over the next two to three years, the operation of the Siderwin unit will simulate the effect of the process on electricity supply.
As Siderwin replaces carbon with electricity, it eliminates the need for agglomeration and coking plants. Instead, the blast furnace would run on electricity and oxygen would be the only gas emitted. Even the formulation process would be simplified. For example, adjustments to the metal’s chemistry (to achieve a specific grade) can be done by adding carbon as the iron plates, produced by electrolysis, are melted.
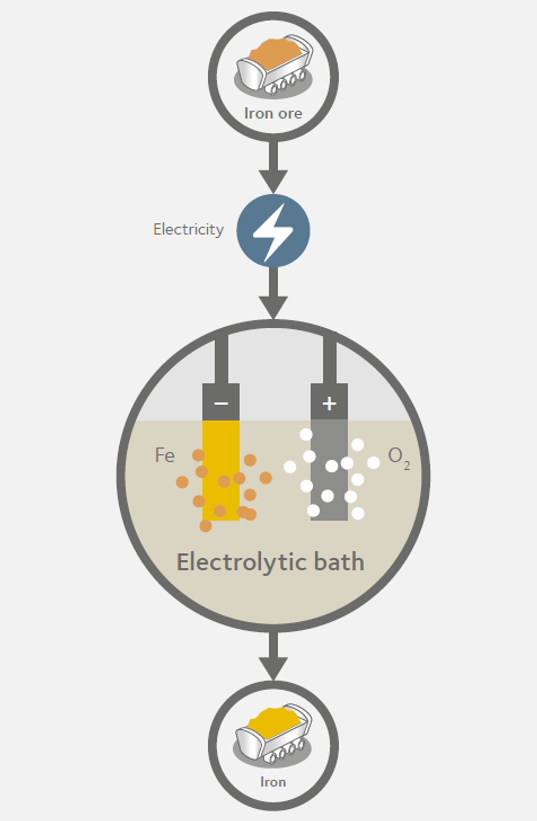
The Siderwin process






